- International News
- Friday-2020-09-04 | 02:57 pm
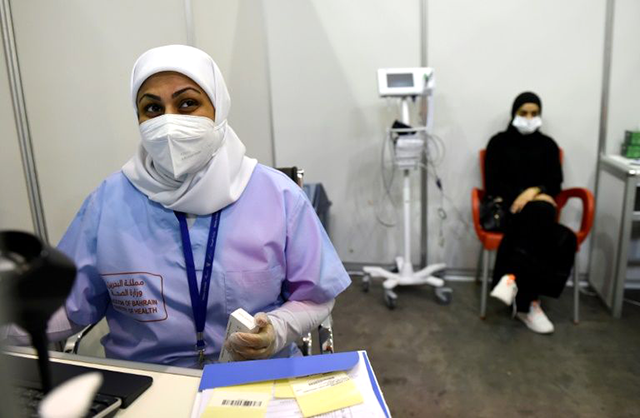
In a Bahrain exhibition centre that used to hold jewellery shows and book fairs before the coronavirus pandemic, Mohammed Al-Blooshi and other masked volunteers wait for a dose of a Chinese trial vaccine.
"It is a service to humanity," says Blooshi, one of thousands in the wealthy Gulf country set to participate in the study.
Chinese drug giant Sinopharm began testing a Covid-19 vaccine in Bahrain in August after starting a similar trial on 15,000 subjects in the nearby United Arab Emirates a month earlier.
The randomised, double-blind trial of 6,000 people is still recruiting healthy men and women as volunteers to test the vaccine's efficacy and safety in a large cross-section of the population.
The trial is due to finish next July, while the overall study is forecast to be completed by September 2021, according to the US National Library of Medicine.
"It's a very small thing to give back to the country," Blooshi says, as other volunteers give blood or fill out forms.
More than 30 potential vaccines are currently being tested on humans across the globe in the hope of ending a pandemic that has now killed more than 850,000 people, according to an AFP tally.
Researchers in the Bahrain study will look at how many patients contract the virus after receiving two doses of the vaccine, as well as examine any adverse reactions.
Novel coronavirus patients are excluded from the trial, as are pregnant women and those with suppressed immune systems.
'Give something back'
Health ministry official Jaleela Sayed Jawad said roughly one-third of the final number of participants had received shots so far.
"Between doses, we will continue to monitor them either by calling them over the phone or, if needed, direct visits," she said.
Bahrain, home to some 1.5 million people, half of them expats, has recorded almost 52,000 novel coronavirus cases, including 190 deaths.
Mohammed Abdulbaqi, another volunteer, said he signed up for the trial "to give something back".
"We hope this pandemic ends and we return to our normal lives," the 25-year-old said as medical teams bustled around the repurposed convention centre in the capital Manama.
Sinopharm executive Liu Jingzhen told Chinese state broadcaster CCTV in July that he had personally been injected with the vaccine.
"We are making smooth progress," he told CCTV, adding that the vaccine "should be available on the market before the end of the year."
In addition to the potential vaccines undergoing clinical trials worldwide, the World Health Organisation is monitoring a further 143 others that are still in the stage of pre-clinical laboratory evaluation.
Upping the ante, Russia on August 11 said it had developed the world's first vaccine offering "sustainable immunity", and was in the final stage of human testing.
In Bahrain, a doctor involved in the trial urged more volunteers to come forward.
"The long hours we have spent treating and testing people all over the country would go to waste if we did not have a vaccination to protect us and the next generation," Haneen Al Boosta told AFP.
"We encourage everyone to stay safe... and to be part of a trial."













